Wayne Lumbasi
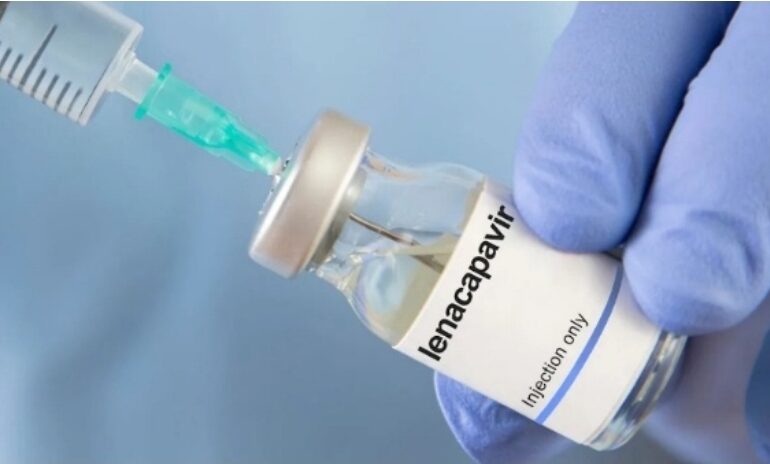
A groundbreaking agreement is set to make a twice yearly HIV prevention injection dramatically cheaper for countries hardest hit by the epidemic. Under the deal, Gilead Sciences has granted voluntary licences to six generic drugmakers including India’s Dr Reddy’s and Hetero Labs to produce lenacapavir, a long acting pre exposure prophylaxis (PrEP) jab.
Currently sold at more than $28,000 a year in the United States, lenacapavir will be available for around $40 per patient per year in 120 low and middle income countries from 2027. Health experts say the agreement could transform HIV prevention by putting one of the most effective and convenient options within reach of millions.
Lenacapavir is injected only twice a year, overcoming one of the biggest challenges of oral PrEP which is daily adherence and reducing the stigma associated with pill taking. The World Health Organization (WHO) endorsed it as an additional prevention tool in July and Europe recently approved it for use in adults and adolescents at risk of infection.
Advocates call the price cut a genuine chance to end new HIV transmissions particularly in sub Saharan Africa which carries two thirds of the global burden. But hurdles remain. Generic production will not start for at least two years, regulatory approval will be needed in each country and many upper middle income nations are excluded from the licence.
Still, the deal marks a turning point for global HIV prevention. If rolled out effectively, the low cost injection could reach key populations including young women, men who have sex with men and sex workers and significantly reduce new infections worldwide.
RELATED
THE SILENT KILLER: HIGH BLOOD PRESSURE IS ON THE RISE








